Test Overview
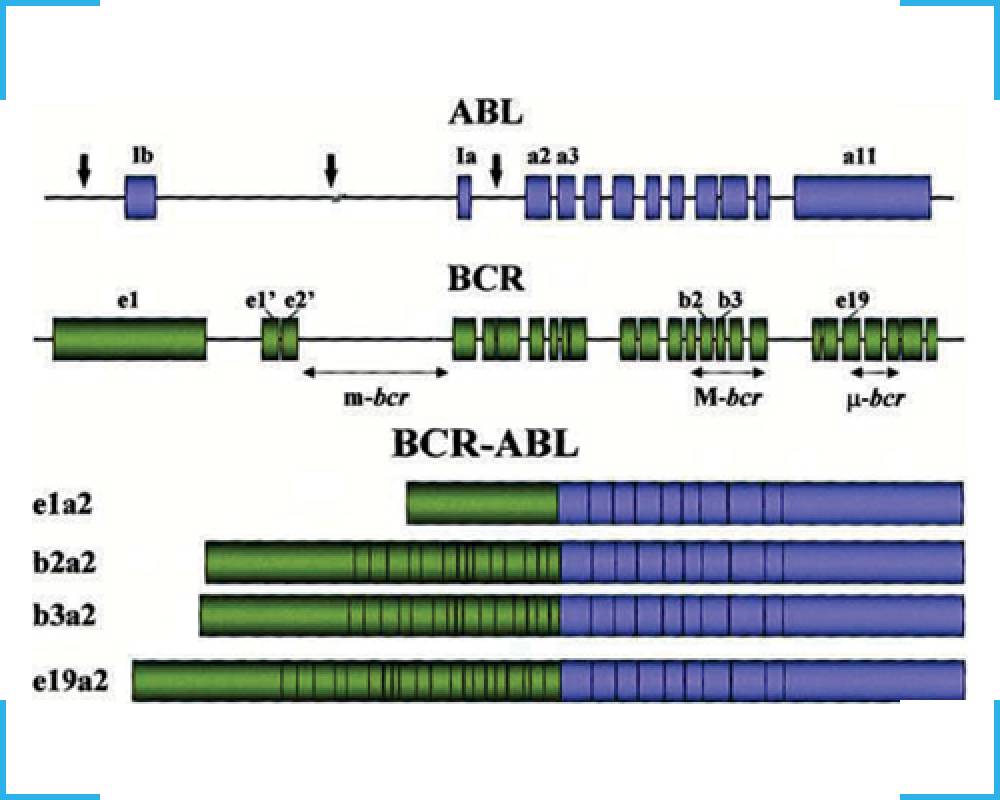
TRUPCR® BCR-ABL QT kit is a RT-qPCR test for the quantitative detection of BCR-ABL fusion transcripts in bone marrow or peripheral blood samples. The kit provides an advantage by detecting, differentiating and quantifying all the three break point cluster regions i.e. major/P210 (M-bcr), minor/P190 (m-bcr) and micro/P230 (mu-bcr) in separate tubes, making it one of the most unique and comprehensive solution currently available..
Principle and Procedure:
It is a two-step protocol in which total RNA from patient’s peripheral blood or bone marrow is isolated, the RT enzyme reverse transcribes total RNA and yields single-stranded cDNA. This is followed with real-time quantitative PCR amplification and quantification of BCR-ABL fusion transcripts and the ABL transcript. Three independent RT-qPCR reactions are performed to detect all the known fusion transcripts in separate tubes to differentiate, quantitate and report individual transcripts.
Key Features:
- The kit is calibrated to report on International Scale using First WHO International Genetic Reference Panel for quantitation of BCR-ABL1 translocation by RQ-PCR (NIBSC code: 09/138) for harmonization of results among laboratories.
- The measuring standards of the kit are calibrated to European reference material ERM-AD623a-f, produced and certified under the responsibility of the Institute for Reference Materials and measurements of the European Commission’s Joint Research Centre.
- The kit allows highly sensitive deep molecular response reporting based on European Treatment and Outcome Study (EUTOS) guidelines.
- Detection, differentiation and quantification of all three Major, Minor and Micro transcripts.
- All inclusive kit: The Assay includes cDNA preparation components and all the PCR components including PCR Pre-mix / mastermix for optimized results. No need to standardize your own cDNA prep which may lead to variability in the results.
Ordering Information:
| CAT. NO. | PRODUCT | CONTENTS |
|---|---|---|
| 3B1267 | TRUPCR® BCR-ABL Quantitative Kit – Major, Minor & Micro | 48 Reactions |
| 3B1268 | TRUPCR® BCR-ABL Quantitative Kit – Major, Minor & Micro | 96 Reactions |
| 3B1253 | TRUPCR® BCR-ABL Quantitative Kit– Major & Minor | 48 Reactions |
| 3B1254 | TRUPCR® BCR-ABL Quantitative Kit– Major & Minor | 96 Reactions |
| 3B1251 | TRUPCR® BCR-ABL Quantitative Kit – Major | 48 Reactions |
| 3B1252 | TRUPCR® BCR-ABL Quantitative Kit – Major | 96 Reactions |
Publications :
- Singh, Neetu, et al. "Differential genomics and transcriptomics between tyrosine kinase inhibitor-sensitive and-resistant BCR-ABL-dependent chronic myeloid leukemia." Oncotarget 9.54 (2018): 30385.
- Chadha, Ritu, et al. "Cytogenetic Risk Stratification of B-Acute Lymphoblastic Leukemia and Its Correlation with Other Prognostic Factors." Indian Journal of Hematology and Blood Transfusion 39.1 (2023): 141-145.
- Dey, Samya, et al. "Deep sequencing reveals the spectrum of BCR-ABL1 mutations upon front-line therapy resistance in chronic myeloid leukemia: An Eastern-Indian cohort study." Cancer Treatment and Research Communications 33 (2022): 100635.