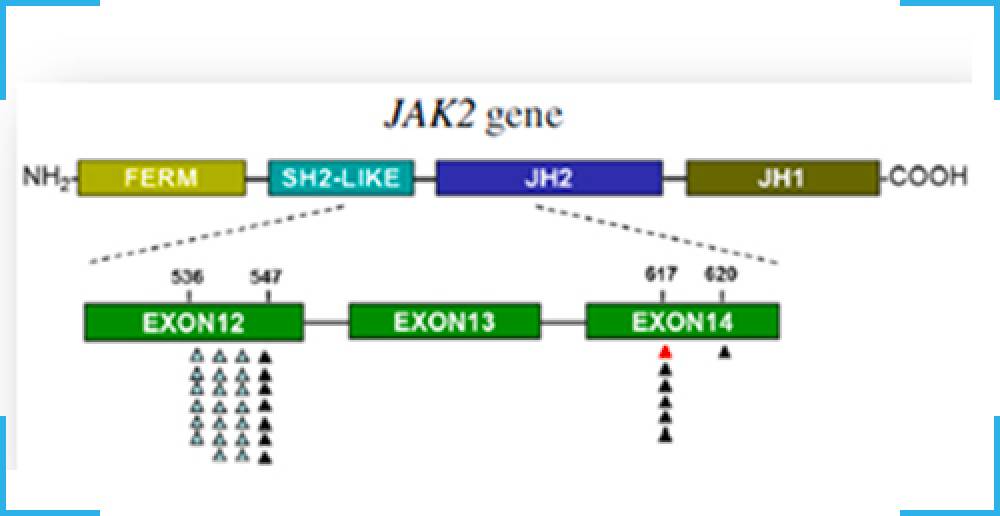
TRUPCR JAK-2 allele burden kit is a real-time polymerase chain reaction (PCR) assay for quantification V617F mutation in JAK-2 (Janus Kinase 2) gene against a background of wild-type genomic DNA. This mutation is a guanine to thymidine transversion in position 1849 of the JAK-2 gene, which leads to a valine to phenylalanine substitution in position 617 of the protein (V617F). The test utilizes mutation-specific primers and a JAK-2 V617F/G1849T targeted fluorescent probe to detect low-copy number (1%) JAK-2 V617F-mutant DNA in cancer tissue.
The TRUPCR JAK-2 V617F allele burden Kit is a highly selective and sensitive assay for quantification of V617F mutation in the JAK-2 oncogene.
Key Features:
- The test allows highly sensitive quantification up to 1% mutant sequence copies in a background of 99% wild-type DNA.
- High specificity due to site-specific hydrolysis probes utilized in the reaction.
- Quantitative standards are calibrated to WHO 1st International Reference Panel for Genomic JAK2 V617F mutation.
- Easy workflow & compatible with most of the real-time PCR equipments.
- Available in different pack sizes and extraction formats.
More About JAK-2
The Janus kinase 2 gene (JAK-2) codes for a tyrosine kinase (JAK-2) that is associated with the cytoplasmic portion of a variety of transmembrane cytokine and growth factor receptors important for signal transduction in hematopoietic cells. Signaling via JAK-2 activation causes phosphorylation of downstream signal transducers and activators of transcription (STAT) proteins (eg, STAT5) ultimately leading to cell growth and differentiation.Chronic myeloproliferative neoplasms (MPNs) are clonal hematopoietic stem cell malignancies characterized by excessive production of blood cells. BCR-ABL1-negative MPN frequently harbor an acquired single nucleotide mutation in JAK-2 characterized as c.G1849T; p. Val617Phe (V617F) and it is a gain-of-function mutation that leads to clonal proliferation. The JAK-2 V617F is present in 95% to 98% of polycythemia vera (PV), and 50% to 60% of primary myelofibrosis (PMF) and essential thrombocythemia (ET). It has also been described infrequently in other myeloid neoplasms, including chronic myelomonocytic leukemia and myelodysplastic syndrome. Diagnostic criteria for ET, MF, and PV adopted by the World Health Organization (WHO) include identification of a clonal marker, with a specific recommendation to test for the JAK-2 V617F mutation in exon 14.1 Detection of the JAK-2 V617F is useful to help establish the diagnosis of MPN and The JAK-2 allele burden decreases with successful therapy, disappears in some patients, and reappears during relapse.
Ordering Information:
| CAT. NO. | PRODUCT | CONTENTS |
|---|---|---|
| 3B1248 | TRUPCR® JAK2 allele burden kit | 24 Reactions |
| 3B1249 | TRUPCR® JAK2 allele burden kit | 48 Reactions |