The TRUPCR® KRAS PCR Kit (K-RAS Kit) is an in vitro diagnostic test intended for the qualitative detection of KRAS somatic mutations in the genomic DNA extracted from fresh, frozen or formalin fixed paraffin-embedded (FFPE) tissue. The TRUPCR® KRAS PCR Kit is based on allele specific amplification and is achieved by ARMS PCR. Taq DNA polymerase is extremely effective at distinguishing between a match and a mismatch at the 3’-end of a PCR primer. The kit is designed to selectively amplify mutant specific sequences in samples that contain a mixture of wild-type and mutated DNA. The most common mutations are found in codons 12, 13 and 61. The detection is achieved using fluorescent probes labelled with FAM and HEX. The TRUPCR® KRAS PCR Kit is composed of 11 assays for the detection of the KRAS mutations and a reference control gene of KRAS region without any known polymorphism / mutation.
Key Features:
- Selective Amplification of DNA containing mutation with ARMS Technology.
- Sensitive to detect up to 1% mutation in KRAS gene.
- Detects 22 different mutations in a single run.
- Extraction control included to avoid false-negative results.
- Rapid, more reliable, comprehensive and cost effective tests.
- Easy work flow & compatible with various Real Time PCR instruments.
KRAS Detectable mutations:
| EXON | CODON | MUTATION | NUCLEOTIDE CHANGE |
|---|---|---|---|
| 2 | 12 | G12C | c.34G>T |
| G12S | c.34G>A | ||
| G12R | c.34G>C | ||
| G12V | c.35G>T | ||
| G12D | c.35G>A | ||
| G12A | c.35G>C | ||
| 2 | 13 | G13D | c.38G>A |
| 3 | 59 | A59T | c.175G>A |
| A59E | c.176 C>A | ||
| A59G | c. 176 C>G | ||
| 3 | 61 | Q61K | c.181C>A |
| Q61L | c.182A>T | ||
| Q61R | c.182A>G | ||
| Q61H | c.183A>T | ||
| Q61H | c.183A>C | ||
| 4 | 117 | K117E | c.349A>G |
| K117R | c.350A>G | ||
| K117N | c.351A>C | ||
| K117N | c.351A>T | ||
| 4 | 146 | A146T | c.436G>A |
| A146P | c.436G>C | ||
| A146V | c.437C>T |
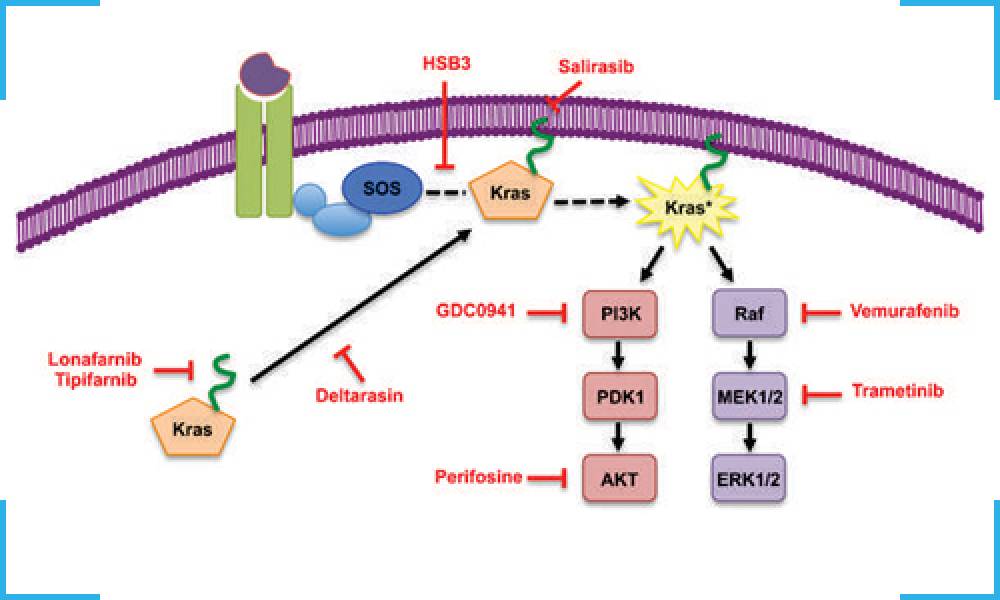
This kit can become useful in suggesting that all patients to be administered anti-EGFR monoclonal antibody therapy, should first be screened for KRAS mutations.
Ordering Information:
| CAT. NO. | PRODUCT | CONTENTS |
|---|---|---|
| 3B1261 | TRUPCR® KRAS Mutation Kit | 48 Reactions |
| 3B1262 | TRUPCR® KRAS Mutation Kit | 96 Reactions |