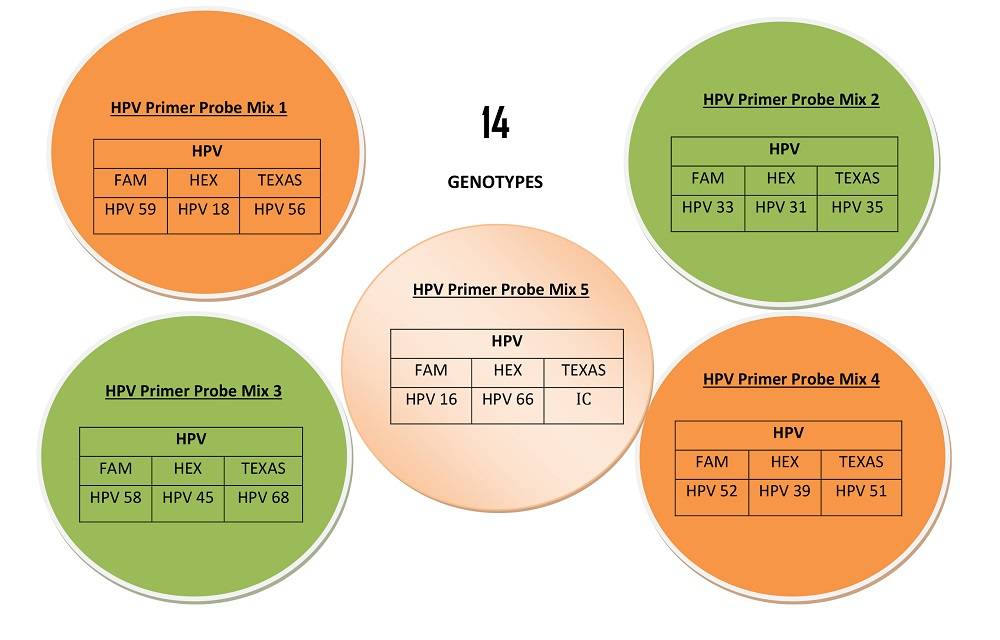
Detection and Genotyping of 14 High Risk HPV Genotypes in clinical samples
TRUPCR® HPV High Risk Genotyping Kit is designed for the qualitative detection of DNA of High-Risk Human Papillomavirus (HPV) in clinical samples by using Real Time PCR. HPV infections are among the most common sexually transmitted infections. Most HPV infections have a benign clinical consequence and are cleared spontaneously. However, persistent HPV infection may result in progression to cervical cancer. More than one hundred different HPV genotypes have been identified, among which over forty infect mucosal and genital epithelia. Genital HPV genotypes are generally classified into high risk (HR) and low risk (LR) groups based on their carcinogenic potential. High-risk HPVs also called oncogenic HPVs, which have been confirmed to cause cancer, includes HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. And some HPVs are possibly carcinogenic to humans, like HPV 26, 53, 66, 70, 73 and 82, that be classified as high-risk or probably high-risk HPVs TRUPCR® HPV High Risk Genotyping Kit is an Real Time PCR assay based on oligonucleotide hydrolysis principle which allows higher specificity and sensitivity of E6/E7 region by primer and probes specific for detection and genotyping of 14 High Risk HPV Genotypes (16/18/31/33/35/39/45/51/52/56/58/59/66 and 68) .
Key Features:
- TaqMan Probe-Based Real-Time PCR Assay.
- Detection and simultaneously genotyping of 14 High Risk HPV Genotypes
(16/18/31/33/35/39/45/51/52/56/58/59/66 and 68). - Based on E6/E7 oncoproteins which mediate the development of cervical cancer.
- Endogenous internal control DNA which allows excluding unreliable results.
- Higher sensitivity and specificity.
- Extensively validated on clinical samples.
- Rapid, reliable, comprehensive and cost-effective test.
- Easy work flow & compatible with with QuantStudio® 3, 5 and 12 series, Rotor-Gene Q , Bio-Rad CFX96, CFX384 , Roche – LightCycler® 480 -II and Applied Biosystems™ 7500.
The role of HPVs in the etiology of cervical cancer is tightly correlated with the over expression of two oncogenes (E6 and E7) due to a specific opening in the E2 open reading frame in the integrated viral genome. The loss of the L1 gene and its impact on viral replication after integration could lead to relatively low viral loads, this suggests that the presence of the E6 and E7 proteins is a specific marker for high-grade lesions detection. Approximately 99.7% of cervical cancers are caused by high-risk HPV infection. Cervical cancer is the second-most frequent malignancy among women worldwide. Compared with cervical screening methods identifying cytological abnormalities, molecular tests that specifically detect the presence of HR HPV DNA in cervical cells can potentially increase sensitivity and cost effectiveness of cervical cancer screening programs.
Ordering Information:
| CAT. NO. | PRODUCT | CONTENTS |
|---|---|---|
| 3B1423 | TRUPCR® HPV HIGH RISK GENOTYPING KIT | 48 Reactions |
| 3B1424 | TRUPCR® HPV HIGH RISK GENOTYPING KIT | 96 Reactions |
Publications:
- Narayanan, Geeta S., M. S. Ganesh, and Rishabh Kumar. "Comparison of treatment response in cervical carcinoma patients infected with human papillomavirus 16 and human papillomavirus 18 who are treated with chemoradiation." Journal of Cancer Research and Therapeutics 17.1 (2021): 204-210.
- Zheng, Ziwen, et al. "Prognostic value of HPV 16/18 genotyping and geminin mRNA quantification in low-grade cervical squamous intraepithelial lesion." Bioengineered 12.2 (2021): 11482-11489.
- Gupta, Shipra, et al. "Burden and associated genotype patterns of high-risk human papilloma virus infection and cervical cytology abnormalities among women in Central India." Infectious Diseases in Obstetrics and Gynecology 2022 (2022).
- Jwala, M., et al. "Prognostic Footprints of HPV Genotyping in Locally Advanced Carcinoma Cervix–A Single Institutional Study." International Journal of Radiation Oncology, Biology, Physics 114.3 (2022): e256-e257.
- John, Julie Hansa, et al. "Study to determine efficacy of urinary HPV 16 & HPV 18 detection in predicting premalignant and malignant lesions of uterine cervix." International Journal of Gynecology & Obstetrics 161.1 (2023): 79-85.
- Kulkarni, Sayali P., Shruti Paliwal, and Susmit Kosta. "Genotypic Diversity of Human Papillomavirus (HPV) Types and Its Prevalence With Cervical Cancer (CC) in Central India." Cureus 15.2 (2023).
- Khullar, Geeti, et al. "High-risk genital-mucosal human papilloma virus types 58 and 59 associated with solitary angiokeratoma on the elbow." Dermatology Practical & Conceptual 13.1 (2023).
- Patel, Sangram Singh, et al. "Comparison of Human Papillomavirus Genotype Detection in Paired Urine and Self-Collected Cervical Swabs: A Pilot Study." Asian Pacific Journal of Cancer Prevention 24.7 (2023): 2427-2430.
- JAYAPRAKASH, DR PARIKSHITH, and DR GEETA S. NARAYANAN. "Role of HPV DNA Testing and its influence on clinical outcomes in Cervical Cancer." Radiotherapy and Oncology. Vol. 133. ELSEVIER HOUSE, BROOKVALE PLAZA, EAST PARK SHANNON, CO, CLARE, 00000, IRELAND: ELSEVIER IRELAND LTD, 2019.